
PathoSeal
The PathoSeal 95 range is designed and tested to withstand a pressure difference of 95 kPa to meet UN3373 Biological Substance, Category B regulations.
The PathoSeal Liquitite range is designed and tested to meet Category B transport regulations when used in conjunction with 95 kPa primaries.
This range of flexible secondary packaging is ideal for the transportation of a variety of primary containers such as blood vials, swabs (including COVID-19 test swabs), blood collection bags and medical devices.
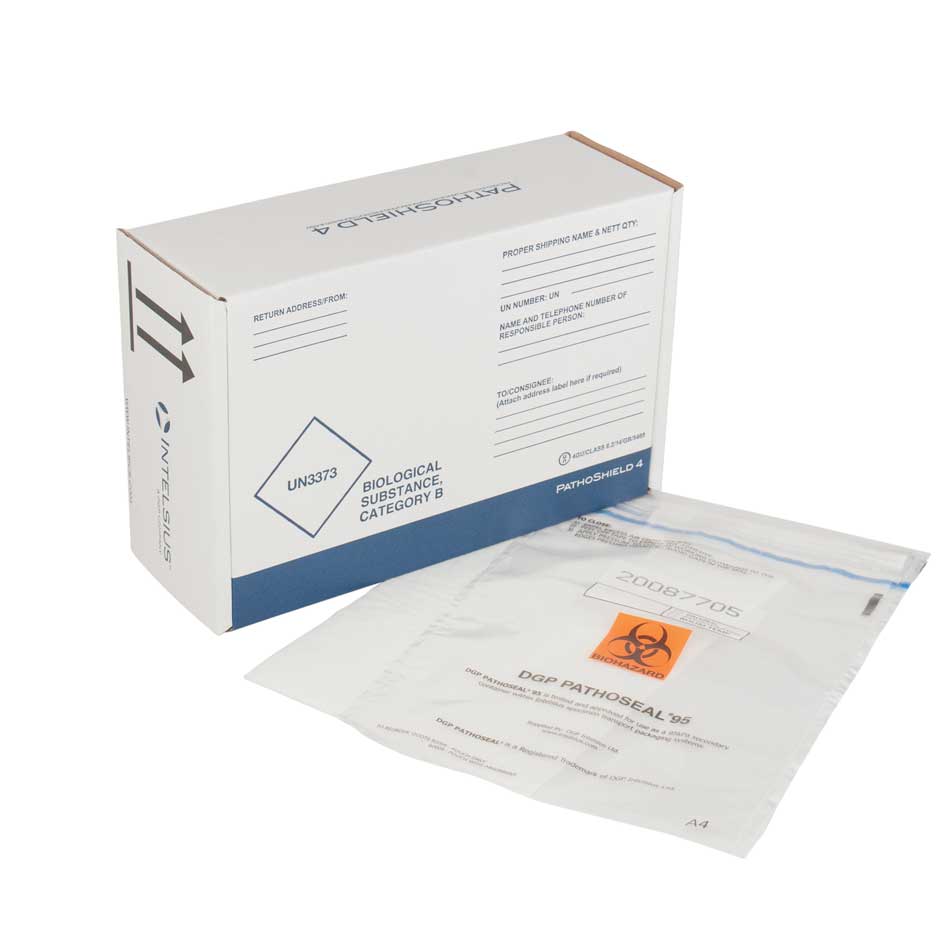
All PathoSeal products feature a simple closure system enabling ease of packing, plus the unique tamper evident seal ensures the integrity of samples. In addition the bags are sequentially numbered, providing a chain of custody and additional security. An external document pouch is also present for all sizes with the exception of PathoSeal 95 A6.
Download Product Sheet
Widely used to support COVID-19 testing schemes
PathoSeal solutions are often used in conjunction with our PathoShield outers, providing a complete UN3373 Category B sample packaging solution that keeps samples safe, secure, and certified prior to testing and sample analysis. Recently our PathoSeal products have played a key role in both public and private Coronavirus testing schemes.
The simple open and close feature and range of size variants means the chosen PathoSeal solution can quickly slot into a current COVID-19 testing scheme or support a new process designed to suit your business requirements. Please call our customer services team now to discuss how the PathoSeal can be the perfect packaging solution to support your COVID-19 testing demands.
