Clinical trials are an essential component of drug research and development, and form the basis of bringing potentially life-saving drugs to the mass consumer market. The trial process is usually a lengthy one, comprising three phases in which drugs are tested several times, using different dosages and on a wide range of patients. The clinical trials market size globally was valued at US$ 80.7 billion in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 6.49% from 2024 to 2030.
In this short article, we will highlight the different phases of clinical trials and why selecting the correct packaging is crucial for the transport of samples to and from testing and healthcare facilities.
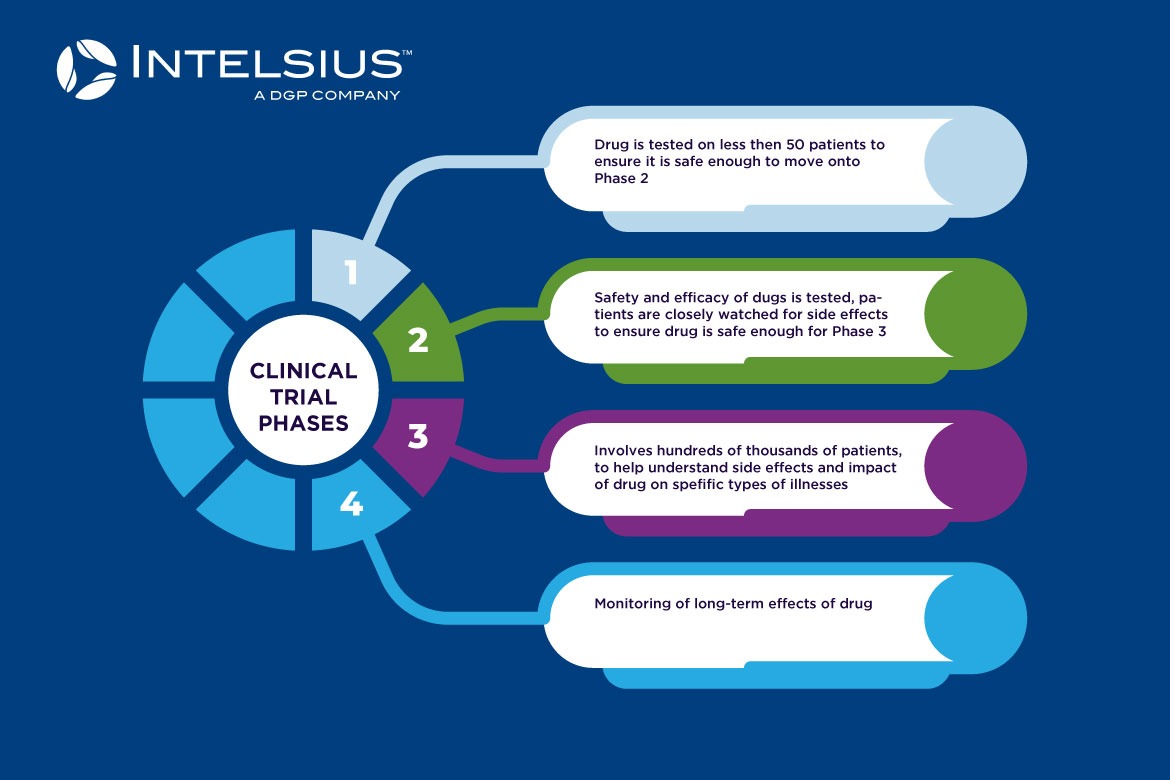
How do clinical trials work?
Clinical trials are usually split into three main phases, with each phase increasing in patient numbers, as clinicians test the new drug or treatment for safety, efficacy and side effects. The development of each phase will depend on the type of treatment being tested however the overarching framework will not differ too much between treatments.
Below is a breakdown of each phase of a clinical trial.
Phase 1:
- Usually involves up to 50 patients, which are divided into groups or cohorts
- Each cohort is given an increased amount of the drug to determine the most suitable and safe dosage for the next phase of testing
- Doctors may collect blood or urine samples to test drug levels in the patients’ systems
- To determine if the drug is safe enough to proceed to Phase 2
Phase 2:
- Involves less than 100 patients
- Patients are closely watched for any side effects
- To determine if drug is safe enough to proceed to Phase 3
Phase 3:
- Involves hundreds or sometimes thousands of patients, some even from different countries
- Usually split into the control group and the study group
- Control group includes patients receiving the standard treatment while the study group receives the new treatment
- To determine if the new treatment works for a specific type of disease (i.e specific type of cancer)
- To gain a deeper understanding of side effects
- How the drug affects patients’ quality of life
Phase 4:
- To test long term side effects of drug
Why the right packaging is important
Throughout the clinical trials, doctors and scientists will need human biological samples like urine, blood, saliva, hair, or even faeces. These samples are taken at different points throughout the clinical trial phases for various reasons including but not limited to: ensuring that the clinical trial is suitable for you, to test for levels of the drug within patients’ system, or to test how the drug or treatment affects patients.
When transporting the above biological samples, selecting the right packaging is crucial to maintain the integrity of the samples as they travel to and from testing and healthcare facilities.
It is vital to select packaging that is durable, compliant, and appropriate to keep the samples safe and intact until its arrival at the final destination. Some samples will also be required to be kept at specific temperatures throughout its journey, which will require packaging that is not only compliant, but is equipped to provide the right amount of thermal protection, particularly if samples travel through different temperatures and levels of humidity.
Compliance
Clinical trials may involve the shipping of infectious or dangerous substances. The International Air Transport Association (IATA) Dangerous Goods classification of infectious substances is divided into Category A and Category B substances, and must adhere to specific packaging and labelling requirements when they are transported. These substances are further assigned a specific UN number depending on which class it falls into.
Besides compliant packaging, infectious sample shipments must also be labelled correctly to maintain the safety of those handling the shipment. Intelsius has created a helpful Label Compliance Guide which you can access here, if you are unsure which labels to use for your shipment.
Intelsius Solutions
Intelsius has been designing and manufacturing compliant sample transport solutions for over 25 years. We offer a range of solutions which we have taken steps to ensure full compliance when shipping clinical samples, including transporting samples for diagnostics right up to delivering pharmaceuticals and medicines to patients.
Pathopak
Certified for Category A and B shipments, the Pathopak range meets ATA, ADR, and CFR 49 (DOT) transport regulations and ensures protection for your samples irrespective of their specimen class. It is a strong and highly durable solution that is suitable for a range of primaries such as blood tubes, specimen containers, swabs, blood collection/transfusion bags and medical devices. It is also reusable and cost-effective.
Intelsius Pathopak solutions are available in four sizes: 800ml, 1L, 2L and 3L and are designed with equal circumference to facilitate ease of packing and removal of samples.
Pathoshield
The Pathoshield range is a complete shipping solution specially designed to carry a range of primaries blood vials, swabs and sample containers. Every solution is pre-printed with the required UN markings and includes the relevant components to transport samples in compliance with transport regulations. The Pathoshield solution is highly flexible is suitable for all stages of clinical trials sample transport and is fully compliant to ship Category A and Category B infectious materials.
Both the Pathopak and Pathoshield ranges have options to add labels, containers, primaries and secondaries to ensure suitability for intricate Phase 1 trials to more extensive Phase 3 trials. Both solutions undergo significant testing, including pressure and drop tests, to ensure their durability and efficacy when transporting infectious samples.
BioTherm
Intelsius’ BioTherm range is fully compliant to IATA, ADR, and 49 CFR (DOT) transport regulations and is available in temperature ranges of 2-8°C, 15-25°C or with dry ice. BioTherm packaging systems are light, durable and pre-qualified for up to 120 hours. The systems are also compatible with a range of data loggers so you can easily track your shipment as it moves across its journey. Learn more about the BioTherm range by clicking here.
ORCA Sample Transport
For a high performance sample transport solution that requires temperature-control, our ORCA Sample Transport (ORCA STP) packaging is fully compliant with Category A and B regulations and is tested against the international ISTA 7D temperature profiles to ensure the highest level of protection for your samples.
ORCA STP combines the PathoPak and the ORCA temperature-controlled packaging system into one complete shipping solution that is also compatible with a range of data logging and tracking devices, so you can have peace of mind when your high-value samples are being transported to their final destination. The solution comes in a range of sizes and temperature profiles including2 -8°C, 15-25°C and dry ice. It is also available as a single use or multi use system.
Get in touch
If you would like to know more about our compliant sample transport packaging solutions or about any of our other packaging solutions, please contact cs@intelsius.com and our customer service department will assist you.
External Sources