The Rise of Remote Healthcare Services
How are new technologies, and more recently, COVID-19 changing the way healthcare services are delivered? We’ll explore the emergence of home deliveries of drugs and therapeutics, remote patient monitoring, and even entire pharmaceutical and vaccine clinical trials now being conducted remotely.
We’ll look at what this means for temperature-controlled and sample transport logistics and whether this new drive towards remote service delivery is changing the way we think about cold chains.
What we’ll cover in this article (click to skip ahead):
- How technological advances and COVID-19 are changing the way we live
- Remote healthcare delivery and patient monitoring
- The rise in home-based patient sample transport
- The rise of personalised medicine home delivery
- The rise in remote, decentralised clinical trials
- How the rise of remote will impact the cold chain
- Intelsius solutions
The Rise of Remote Living
Long before COVID-19 forced many industries to reconsider set-in-stone working practices, making way for more flexible, remote-style working patterns, technological advances were driving a change in the way we conduct our lives. The internet has paved the way for more flexible social and working practices for the last three decades, but it’s the more recent development of smart devices, programmes, and services that have seen many of us change the way we live and work.
The last decade before COVID-19 (2009 – 2019) saw a 27 per cent rise in home working,1 and while remote working, home deliveries, and a more home-based lifestyle aren’t recent phenomena, both technological advances and, more recently, COVID-19 have driven a significant shift towards a home-based lifestyle.
High-speed internet, smartphones, tablets, high-quality home computers, and the creation of community video platforms and apps have changed the way we think about work and play. COVID-19 has accelerated the shift towards a more remote lifestyle, with the number of people home working more than doubling since the pandemic began, with home-working now the norm for over a quarter of the U.K. workforce.2
But this shift towards remote hasn’t been limited to work. According to the ONS, 82 per cent of U.K. internet users shopped online in 2019, up from 53 per cent in 2008.3 During the COVID-19 pandemic, online shopping grew by 75 per cent in the U.K.4 Whether it’s our weekly food shop, clothing, or electronic devices, there’s little if not nothing we can’t have delivered to our door in a matter of days.
Beyond work and shopping habits, online streaming services such as Netflix and Amazon, online gaming platforms, and apps such as WhatsApp, Twitter, and Instagram mean we can often find what we used to get from visiting public spaces in our own homes. But what, if anything, has this reconfiguration of work and life behaviours meant for the healthcare and pharmaceutical sectors?
Remote Healthcare Delivery and Patient Monitoring
Long before the COVID-19 pandemic, virtual consultations with your doctor were on the rise, with many healthcare systems operating a digital-first approach to service delivery.5 With the arrival of COVID-19, this move has been accelerated. Between February and May 2020, the NHS reported a 34 per cent increase in remote, non-face-to-face GP appointments.6
Dr Richard Vautrey, the GP committee chair at the British Medical Association, said: “There has been a massive change from the vast majority of consultations in general practice taking place face to face, to now the vast majority taking place by telephone or increasingly by video consultation.”7
The NHS has given numerous apps the green light to support this online triage approach. As of July 2020, the NHS had approved 34 apps and online triage platforms to support their delivery of remote consultations.8 Patient and healthcare staff safety, and a desire to reduce stress on healthcare services, coupled with the emergence of trusted technologies and a global pandemic, seem to be changing the way these services are delivered and will continue to be delivered long after COVID-19.
Speaking to your GP via video link to establish symptoms and potential next steps is one thing, but how is this move impacting the physical movement of critical patient samples from the patient to the lab?
Patient Sample Transport
A core part of the patient diagnosis and monitoring process is the collection and testing of human samples. Whether it’s saliva, urine, faeces, or blood, correctly gathering these samples and delivering them securely to relevant test sites is a crucial step in delivering healthcare services.
Throughout the COVID-19 pandemic, home testing kits have been an essential part of monitoring the disease’s spread. Many of us take multiple tests per week and then send them in UN3373 compliant sample packaging for testing. Previously it was common for these tests to be carried out at your local doctors’ surgery or a specialist test centre, where only trained staff were trusted with the collection and delivery of potentially harmful samples.
But even before the advent of COVID-19, home testing for a wide range of conditions was becoming an established part of NHS and other private healthcare providers’ working practices, with detailed guidelines and support for correctly gathering, packaging and sending your samples published on the NHS website.
As well as national healthcare providers, private home testing companies have been springing up throughout the last decade, offering home testing kits for a wide variety of conditions. These are grouped into three kinds: blood tests, DNA tests, and microbiome analysis. For anywhere between £60 and £300, you can receive information about a wide range of existing conditions or conditions likely to develop in future.9
But COVID-19 has been a critical driver in a sharp rise in the use of home testing kits. LetsGetChecked, a leading personal health testing and insights company that offers more than 30 at-home testing kits for coronavirus, STDs and vitamin deficiencies, reports that sales have boomed since the onset of the COVID-19 pandemic in March, recording 880% year-over-year growth from 2019.10
As home testing becomes the norm for almost all conditions and health concerns, the infrastructure that supports it – packaging, testing implements, sample cold chains etc. – will need to grow with it.
Home Delivery of Prescription Drugs
Home delivery of personal medicines is also on the rise, even before COVID-19 restricted access to central medical and pharmacy locations. This shift was underlined by Amazon’s $753 million purchase of the online prescription drug delivery platform, PillPack in 2018. Two years later, in 2020, Amazon launched Amazon Pharmacy, its online and mobile prescription medication ordering and fulfilment service.11
In the way we have batteries, clothes hangers, cutlery, phone chargers and cat food delivered via the Amazon app, we can now have a wide array of personal medicines delivered directly to our door, without any need for face-to-face interactions with a pharmacist. Amazon’s service even comes with a 24/7 chat portal, where users can chat directly to a pharmacist about their prescriptions via a secure video link.
But it doesn’t stop with Amazon. The NHS, Boots, Lloyds Pharmacy and many more now operate home delivery services, all of which require access to compliant and secure temperature-controlled packaging solutions to be shipped in local courier networks (or last-mile cold chain logistics).
As far back as 2017, a report – published by delivery firm CitySprint Healthcare – found that the demand for pharmacies to deliver medicines to patients’ homes has risen in the past year (2016-2017), increasing the pressure on pharmacies to offer ‘retail-style convenience’.
Of the 350 pharmacists surveyed – working across independents, ‘chain’ pharmacies, and in ‘clinical’ pharmacy settings – 79 per cent said their pharmacy business offers home deliveries.
Of these pharmacists, 84 per cent reported an increase in demand for home deliveries in the past year. Of all respondents to the survey, 62 per cent said the most common reason patients request medicines delivered is because poor health prevents them from getting to the pharmacy, while 34 per cent said it was the ‘convenience of not having to come to the pharmacy.12
Fast-forward to 2020, and U.K. pharmacists reported a further 84 per cent rise in home delivery requests (due to COVID-19), with over half (57 per cent) of pharmacists in the UK either being unclear about the legislation surrounding the delivery of medicines to patients’ homes or are not aware of whether there is legislation at all. This suggests there is still work to be done to make guidance clear and accessible – and ensure it is implemented.13
Remote Clinical Trials
The last and perhaps most significant change to established service delivery is a move towards remote clinical trials. The previous six years have seen a sharp rise in the number of clinical trials carried out. According to ClinicalTrials.gov, the number of registered trials has almost doubled from 181,238 trials in 2015 to 325,817 trials in 2020.14 This rise in the number of trials being carried out had already begun the process of digitisation and changes in the approach to participant data gathering.
Before the pandemic, most clinical trials were delivered from a central location, asking participants to attend one or multiple locations so they can be monitored for the duration of the trial. But COVID-19 forced the sector to rethink its approach to trial delivery, with many now being conducted entirely remotely. In 2020, 76 per cent of more than 200 clinical trial sponsors said they conducted most or all their patient monitoring remotely, up from only 18 per cent of respondents the previous year.15
So how does it work? In large part, it’s thanks to modern technologies such as iPads and Bluetooth that enable large-scale clinical trials to be carried out from people’s homes. ‘Participants … are gifted an iPad for filling out daily questionnaires and Bluetooth-enabled technology for recording their own vitals. These gadgets include a pulse oximeter to measure oxygen saturation in the blood, a scale, a thermometer, and a spirometer to measure breathing. On four separate occasions over the course of a month, a phlebotomist comes to the patients’ homes to draw blood and take nasal swabs for COVID-19 testing.’16
With yet another home-based solution being enabled by modern technology and accelerated by COVID-19, remote clinical trials seem here to stay. As with remote patient monitoring, sample gathering and medicine delivery, the successful long-term delivery of large-scale, remote clinical trials will require the support and expertise of packaging and cold chain logistics experts to ensure compliant transportation of human samples within local and national cold chain networks.
What Does This Mean for Cold Chain and Sample Transport Logistics?
With more samples and temperature-sensitive materials being shipped than ever, and with home deliveries and remote practices only likely to increase as new technology and services continue to flood into the market, ensuring local, national and international cold chain networks are functioning and compliant will require the collective efforts and expertise of the entire cold chain logistics sector.
You can read our article about how a cold chain works and what to consider before shipping temperature-sensitive materials here. If you have any questions about cold chain logistics or need support with the shipping of your samples or temperature-sensitive materials, click here to speak to a member of our team.
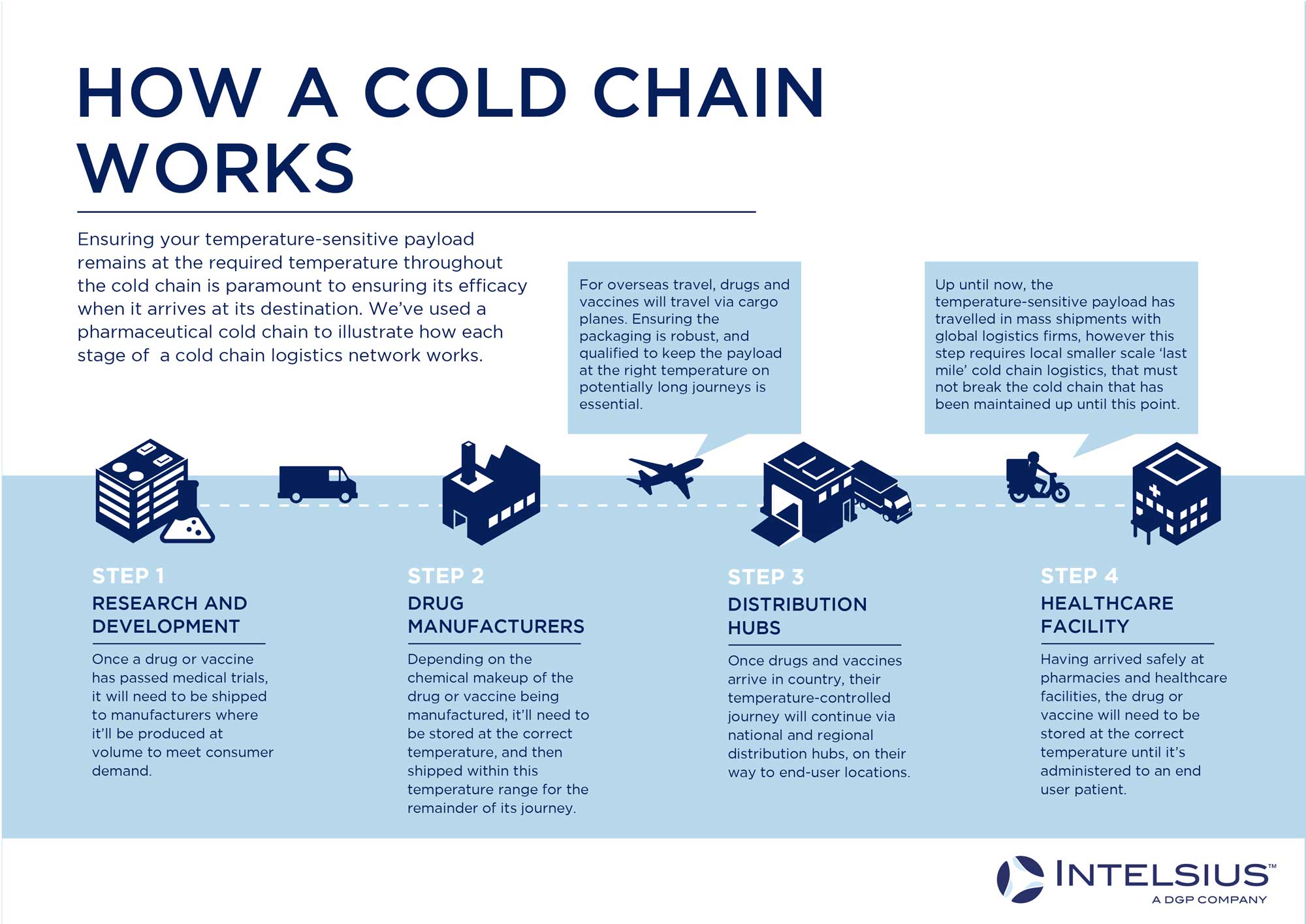
Unlike mass vaccine or pharmaceutical delivery from manufacturing sites to national distribution depots, personal sample gathering and medicine delivery require more local level courier networks, with a focus on smaller, lower volume package deliveries to multiple local destinations.
As the above infographic outlines, a functioning last-mile cold chain logistics network relies on numerous smaller, local courier networks with cold chain capabilities. You can read more about the specific challenges of last-mile cold chain logistics in our article here.
When it comes to packaging, personalised medicines, single or low volume sample collection, and home-based clinical trial sample collection will all require smaller, easy-to-handle temperature-controlled and UN3373 compliant sample transport solutions. In many cases, these packaging solutions will be handled by patients themselves, which will increase the need for easy-to-use solutions that can be handled with a minimum of fuss in a domestic setting.
It’ll also mean an increase in cold chain packaging couriers. Just as apps such as Amazon, Ocado, Deliveroo etc., have all driven demand for drivers, the explosion of personal medicine home deliveries and sample collection will require an increase in trained couriers. This again increases the need for both sample transport and temperature-controlled packaging solutions to be user-friendly and portable, as well as Category A and B compliant.
Intelsius Technical Director Jens Mangelsen expands on what this means for the cold chain: ‘in 2019, the FDA highlighted new supply chain and manufacturing challenges of personalised medicine, and in March 2020, the FDA recommended home treatment of patients in clinical trials for the first time if this does not result in additional risks for the patients. These new medical supply chains have fundamentally changed the requirements for transport solutions, and the pandemic has only accelerated the trend towards home nursing and decentralised clinical trials and, with it, the demand for qualified packaging solutions with end-to-end visibility functionality that meet the latest medical supply chain requirements for such applications.’
To speak to a member of our dedicated team about how we can support your cold chain with temperature-controlled packaging and sample transport solutions, simply click here.
Or, to learn more about achieving UN3373, UN2814, and UN2900 Category A and B compliance, you can find our helpful guide here.
Intelsius Solutions
An ISTA-certified laboratory, we’ve been designing and manufacturing UN Category A and B compliant sample transport and temperature-controlled packaging for over 20 years.
We have a wide range of packaging solutions designed specifically for human samples, vaccines, pharmaceutical and biopharmaceutical products across local, last-mile courier networks.
Whether it’s an off-the-shelf solution or working with our expert Technical Team, we’ll have a packaging solution that can help you deliver remote healthcare services or conduct remote clinical trials.
Click the relevant market below to discover more about how we can meet your specific sample transport or cold chain needs:
- Blood transport
- Biotechnology and pharmaceutical
- Clinical trials
- COVID-19
- Laboratory research and development
- Human and animal samples
- Food testing
Alternatively, you can go directly to our temperature-controlled packaging solutions or sample transport solutions by clicking the relevant buttons below.
Temperature-Controlled Packaging
Get in Touch
To discuss any of the topics covered here further, or enquire about any of Intelsius’ sample transport or temperature-controlled packaging solutions, simply get in touch with your local office by clicking the button below.
External References
- TUC (2019) – Homeworking up more than a quarter in last decade, TUC analysis shows
- The Guardian (2020) – Most people in UK did not work from home in 2020, says ONS
- Office for National Statistics (2019) – How our internet activity has influenced the way we shop: October 2019
- Statista (2021) – Percentage change in online purchases due to the coronavirus (COVID-19) pandemic in the United Kingdom from March 2020 to February 2021
- British Medical Journal (2020) – Video consultations in primary and specialist care during the covid-19 pandemic and beyond
- The Guardian (2020) – GP appointments by phone and video surge during coronavirus lockdown
- The Guardian (2020) – GP appointments by phone and video surge during coronavirus lockdown
- The Health Foundation (2020) – How has COVID-19 affected service delivery in GP practices that offered remote consultations before the pandemic?
- The Guardian (2018) – Blood, spit and swabs: can you trust home medical-testing kits?
- Digital Media Solutions (2020) – New Online Shopping Trend: At-Home Medical Testing
- Techno Crunch (2020) – Amazon launches Amazon Pharmacy, a delivery service for prescription medications
- Chemist and Druggist (2017) – Demand for pharmacy delivery services grows, survey finds
- Healthcare Global (2020) – Home delivery of medication has risen by 84%, report finds
- Florence (2021) – Why Remote Clinical Trial Monitoring is the New Standard
- The Scientist (2021) – Pandemic Accelerates Trend Toward Remote Clinical Trial
- The Scientist (2021) – Pandemic Accelerates Trend Toward Remote Clinical Trials