Category A Compliant
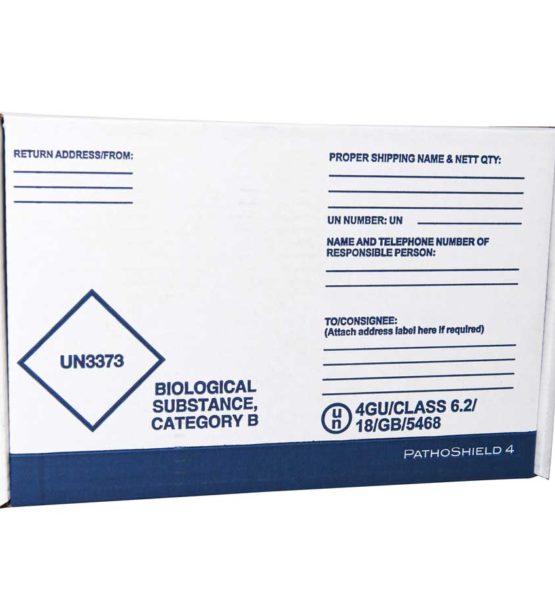
Category B Compliant
Postal Kits
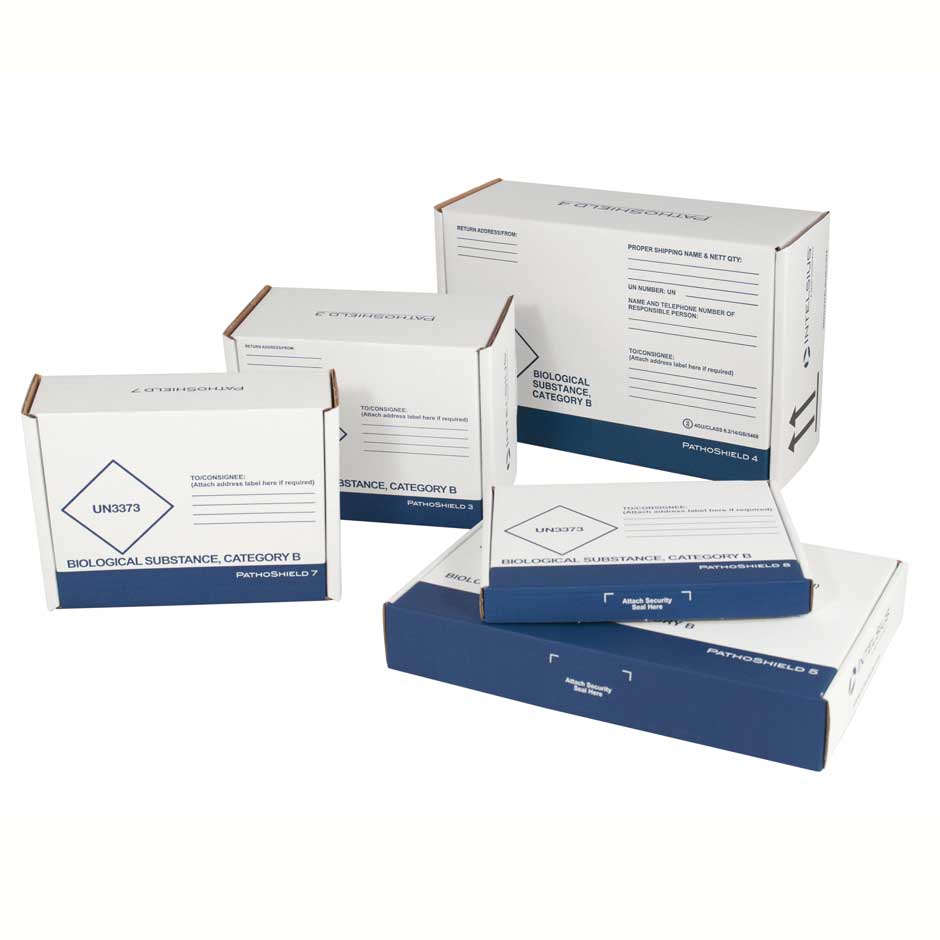
PathoShield
The PathoShield range are complete shipping solutions designed to carry a wide range of primaries, including blood vials, swabs (including COVID-19 swabs) and sample containers. Each system is pre-printed with the required markings and includes the relevant components to ship samples in compliance with transport regulations.
These systems are ideal for use throughout all phases of a clinical trial with ease of shipping and compliance at the forefront of design. Highly flexible in composition these systems can be suitable for transporting a wide range of samples or testing kits.
Download Product Sheet
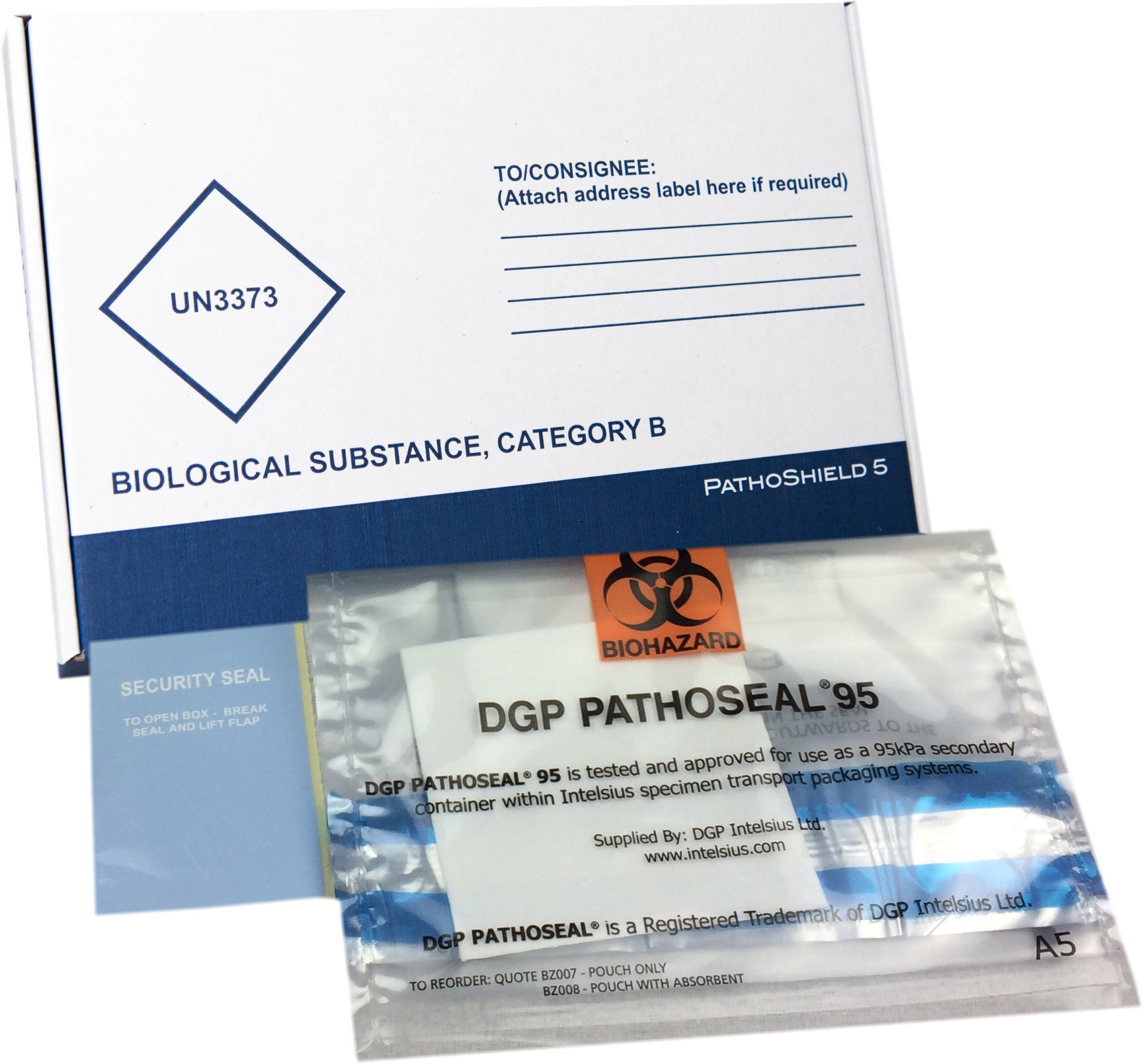
PathoShield packaging solutions are fully compliant and certified to transport Category B and Category A infectious biological materials. Samples being sent for COVID-19 testing and analysis are recommended by the World Health Organisation (WHO) to be classified as Category B UN3373 biological materials. To discuss how to transport your samples in accordance with compliance, please email compliance@intelsius.com or contact a member of our customer services team.
PathoShield Schematics
Complete Category A and Category B solutions include flexible 95kPa tested compliant secondary container, absorbent, labels, and UN markings
Range of sizes suitable for shipping through postal and courier networks
Carry a wide range of primaries including blood vials, swabs and sample containers
Can be supplied as an insulated pack
Supplied flat packed for efficient storage and shipping prior to use