The global cell and gene market, valued at USD 9.95 billion in 2023, is a rapidly expanding industry expected to grow to USD 106.03 billion by 2033. Data for the third quarter of 2023 revealed over 1,800 cell and gene clinical trials being conducted globally with a total investment of USD 2.2 billion. Of the 1,804 trials, North America saw the most ongoing trials, at 940, followed by 747 in Asia Pacific and 340 in Europe.
As the industry is still in its early stages, there are certain specific complexities when organising logistics for cell and gene therapies. In this article, we will look at the logistics challenges faced throughout the cell and gene therapy clinical trial cycle and the main considerations that cell and gene therapy developers and manufacturers must be aware of.
What is cell and gene therapy?
Cell therapy and gene therapy are separate and distinct treatments. Gene therapy involves introducing normal genes into cells to replace “defective” genes in order to treat genetic disorders. Cell therapy involves transferring live cells into a patient to treat diseases. The cells can be from the patient (autologous cells) or a donor (allogeneic cells).
The main difference between cell and gene therapy is that gene therapy involves the transfer of genetic material in a carrier or “vector” and the insertion of that gene into a patient’s cells, while cell therapy involves the insertion of modified cells into the patient. While cell and gene therapy are separate, they are often used together, in the case of stem cell treatment, where stem cells are genetically modified and reinserted into the patient.
Like personalised medicine, cell and gene therapies are highly specialised and catered for the individual patient’s requirements. When dealing with such specialised treatments, there is an inherent need for specific and made-to-order logistics services to meet the demands of the industry. The ability to track and manage data throughout the payload journey from the warehouse to the final destination will be a key way forward to ensuring the safe and secure transit of cell and gene therapies.
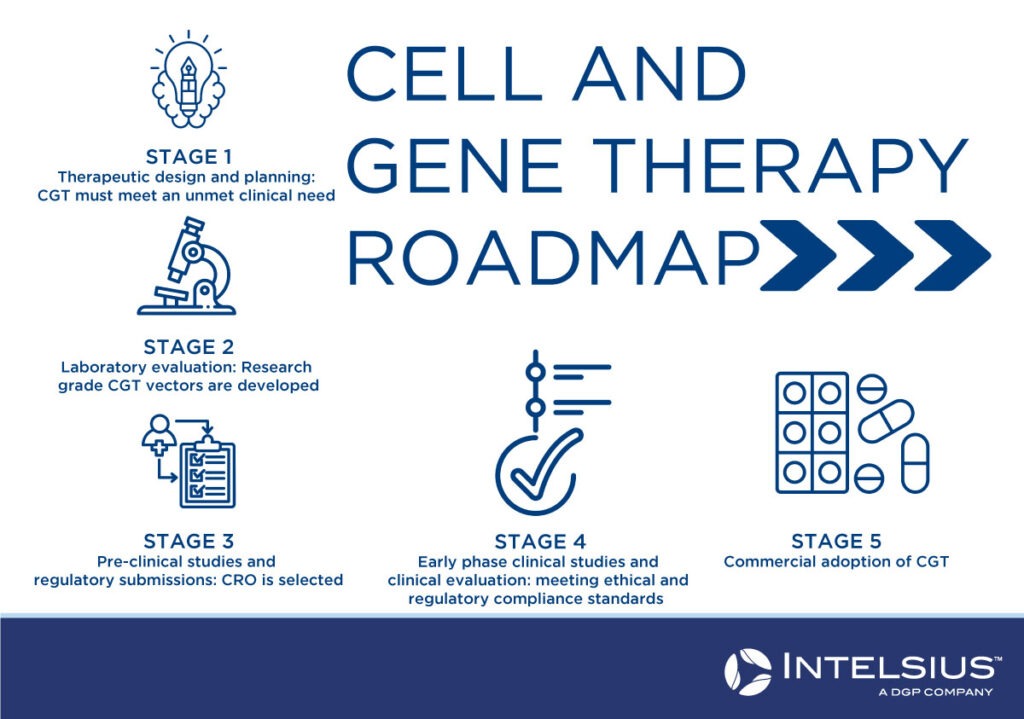
What does the roadmap of cell and gene therapy look like?
Stage 1: Design and planning
To determine the benefit of the proposed cell and gene therapy over currently available treatments and to assess key technical, scientific and medical information needed to meet the requirements of regulators and those funding the therapy. Financing is a key consideration to ensure sufficient funding for the whole project, including research, production and staffing costs. At this stage, intellectual property concerns and applications for “orphan drug” status are also addressed.
Stage 2: Laboratory evaluation
Research-grade cell and gene therapy vectors must be developed to ensure the vector is stable, meets all essential requirements, and meets the targeted product profile. It must also be sufficient for preclinical evaluation. The potential therapy must comply with regulatory guidelines and requirements at this stage.
Stage 3: Pre-clinical studies
A contract research organisation (CRO) will be enlisted to conduct research on a contractual basis to assist with clinical research, clinical trial arrangements, and regulatory affairs. At this stage, an application for a clinical trial is submitted to the relevant governing body.
Stage 4: Early phase clinical studies
At this stage, regulatory and ethical compliance are achieved so that Phase 1 and 2 clinical trials can be carried out. While Phase 1 clinical trials are to determine the safety of therapy, Phase 2 trials will focus more on its efficacy in the patient population.
Stage 5: Commercial Adoption
The final stage of CGT approval will involve Phase 3 and Phase 4 clinical trials to test the efficacy and safety of treatment on a wider population sample group. Commercial manufacturing processes are nailed down during this stage, with eventual adoption into the national health system and/or for public consumption.
Throughout the drug discovery process, patient cells will be transported back and forth, sometimes to multiple healthcare testing sites, before being delivered to the manufacturer. As live cells must remain at specific temperatures throughout transit to maintain their integrity, cold chain logistics, packaging and storage are key factors that must be considered carefully.
What part do temperature-controlled logistics play in cell and gene therapies?
Main considerations for cell and gene therapy logistics:
- Connected logistics
- Regulations compliance
- Selecting the right packaging
- The complexity of supply chains.
Connected logistics
The role of cell and gene therapies is to treat rare diseases. Clinical trials for rare diseases will undoubtedly be spread across different locations and even continents, a challenge requiring an extremely well-connected and stable logistics network with supply chain partners working together to ensure proper handling and delivery of temperature-sensitive cargo. To address this challenge, cell and gene therapy developers must partner with logistics providers with multiple logistics hubs in strategic locations to meet service demands and mitigate cold chain risk.
Regulations compliance
Legislation and guidelines surrounding imports and exports of pharmaceutical products are another complex area to consider. Aside from individual cell and gene therapy transport regulations, compliance requirements for pharmaceutical goods by international bodies like IATA for air transport or ADR for road transportation must be met adequately. These regulations can vary greatly depending on region and location, and specific attention must be paid to meeting these requirements.
The right packaging
Transporting cells and genetic treatments require specialised packaging that can maintain freezing or cold temperatures regardless of the external environment. Payloads are transported across various terrains, temperatures and humidity levels for varying durations, so it is essential to utilise packaging with the appropriate level of thermal protection to ensure the integrity of the samples throughout the journey. Connected packaging with adequate tracking and monitoring capabilities would be essential to ensure payload visibility during shipment so that necessary actions can be taken to prevent temperature excursions.
Complexity of supply chains
Since cell and gene clinical trials cross many countries and regions, the complexity of supply chains and cold chain infrastructure in different locations would present a considerable challenge. An example would be in developing countries and regions where cold chain infrastructure and logistics are not as robust or secure. In these scenarios, temperature excursions become much more common, and the possibility of payload damage becomes more pressing.
This kind of supply chain complexity makes it difficult to generate economies of scale, making creating a sturdy and stable cold chain logistics network much more difficult. Mitigating risks along the supply chain has become more complex and requires much more specialised care and expertise. Data collection, management and automation will be the way forward to address this. Cell and gene developers, manufacturers and logistics players will need to create a seamless network where information can be easily accessed, shared and stored to maintain supply chain visibility.
How can Intelsius solutions serve the cell and gene therapy industry?
High-Performance ORCA Range
Intelsius’ ORCA range utilises vacuum insulated panelling (VIP) combined with advanced phase change materials (PCM). It is specially designed to transport high-value payloads like stem cells and genetic material at low and frozen temperatures. The ORCA range is split into five distinct product types, spanning temperature ranges from -70°C with dry ice, 2-8°C and 15-25°C to meet your specific payload’s temperature needs and is tested and qualified in our ISTA-certified laboratories, guaranteeing up to 120 hours of thermal protection. Sizes range from the handheld ORCA Response to our fully collapsible ORCA Pallet solution. The range also features single-use and multi-use options to cater to all your supply chain requirements.
Connected Packaging Solutions
ORCA Connect is our connected packaging solution compatible with a wide range of data loggers so that you can efficiently and effectively track your payload as it moves across its journey. ORCA Connect allows you the flexibility to track your shipment the way you want to, with real-time tracking capabilities to monitor key data like external and internal temperature, location, orientation, shock and light throughout the payload’s journey.
Customers can also use our universal logger portal, ORCA Cloud, which allows them to track and report on multiple data loggers anywhere in the world, offering total visibility of their payload without needing multiple portals. The easy-to-navigate portal offers customers a seamless way to record all key data in one location, particularly when they have multiple payloads that need tracking across multiple locations – such as during a clinical trial. This service is particularly useful as a complete solution to manage and monitor high-value payloads such as those used in cell and gene therapies.
Global Manufacturing Footprint
Having been in the cold chain packaging solutions industry for over 25 years, Intelsius now has a presence in multiple continents and locations. Our products and services are currently available in strategic locations in the UK, Europe, Asia, Oceania, Africa and North and South America. We also have offices and distributors in strategic locations worldwide, the full list of which you can access here. Our global manufacturing footprint is part of our strategy to reduce customer costs, mitigate supply chain risks and reduce our carbon footprint.
Get in touch
If you would like to know more about our Intelsius branches, our other temperature-controlled packaging solutions, or how we can support your cell and gene therapy requirements, contact cs@intelsius.com. Our customer service representatives will be happy to assist you.
External Sources
- https://www.ucl.ac.uk/therapeutic-innovation-networks/education-centre/cell-gene-therapy-translation-roadmap
- https://www.biocair.com/en/knowledge/thought-leadership-technical-articles/the-challenges-of-cell-and-gene-logistics
- https://cerbaresearch.com/app/uploads/2023/08/CellGene_Viroclinics-DDL-1.pdf
- https://www.labiotech.eu/partner/cold-chain-logistics-cell-gene-therapy/
- https://alliancerm.org/wp-content/uploads/2024/01/20231220_Sector-Snapshot-Outline-Fall-2023_V2.pdf
- https://www.asgct.org/global/documents/asgct-citeline-q4-2023-report.aspx#:~:text=In%20Q4%2C%20the%20number%20of,approvals%20for%20COVID%2D19%20vaccines.