Following on from our long-read piece on the wider environmental impact of COVID-19, in this article we’ll focus specifically on the vaccine cold chain; how each of the currently available vaccines need to be transported, what the implications are for our environment, and how we can limit the damage with intelligent design and sustainable solutions.
To illustrate this, we’ll look closely at the three existing phase 3 trial approved Pfizer-BioNTech, Moderna, and Oxford-AstraZeneca vaccines, and how their unique makeups may influence cold chain logistics with varying environmental implications.
A closer look at the three COVID-19 vaccines currently available
At the time of writing this article there are three COVID-19 vaccine developers that have reported successful phase 3 trials: Pfizer-BioNTech, Moderna, and Oxford-AstraZeneca. In the case of the Pfizer-BioNTech vaccine, the UK began vaccinating with it as of 8/12/20, with more countries ready to begin mass vaccination programs imminently. Each of these vaccines requires different storage and transportation (cold chain) conditions, and each comes with its own unique challenges.
In order to focus specifically on the different environmental challenges facing these three vaccines, we’ll start by looking at each in isolation – their biopharmaceutical makeup, temperature requirements, and individual distribution and logistical challenges.
Pfizer-BioNTech
The Pfizer-BioNTech vaccine is known as a messenger RNA (mRNA) vaccine, which uses the virus’ genetic code rather than any part of the virus itself and is injected into the body where it enters cells and tells them to create antigens. Unlike conventional vaccines, the Pfizer-BioNTech vaccine is synthetic and doesn’t use any of the actual virus (usually a much-weakened form) and is therefore considered by some to be safer than a traditional vaccine.1
A well reported feature of the three vaccines to have passed phase 3 trials is that they will require two doses, given two weeks apart. The extra dose presents additional logistical challenges, as it doubles the amount of cargo and transportation required to immunise the population and requires an extra visit to a designated vaccination centre for every recipient. With this in mind, it’s especially important we think about the reusability of our packaging solutions. And, with the prospect of the COVID-19 vaccination program becoming an annual or twice-yearly necessity, ensuring we have sustainable solutions in place is vital.
Lastly, the Pfizer-BioNTech vaccine requires deep frozen storage (-70°C) to maintain its efficacy. Once thawed, the vaccine can be kept at refrigerated temperatures (2°C – 8°C) for up to 5 days before losing its efficacy. The deep-frozen requirements mean a huge demand for dry ice, and deep-freezing capabilities throughout the cold chain – including in end user facilities such as hospitals, clinics, and temporary vaccination hubs.
Oxford-AstraZeneca
Oxford-AstraZeneca’s vaccine is different from the Pfizer-BioNTech and Moderna vaccines. The Oxford vaccine is a genetically modified common cold virus that used to infect chimpanzees. It has been altered to stop it causing an infection in people and to carry the blueprints for part of the coronavirus, known as the spike protein. Once these blueprints are inside the body, they start producing the coronavirus’ spike protein, which the immune system recognizes as a threat and tries to squash it.2
Another crucial difference between this vaccine, and the Pfizer-BioNTech vaccine is that it only requires refrigerated temperatures (2°C to 8°C), meaning significantly less challenges throughout the vaccine cold chain. This is especially important is less well-connected parts of the cold chain in rural, and economically less affluent parts of the world that are often hotter and have less robust cold chain networks in place.
Moderna
Like the Pfizer-BioNTech vaccine, Moderna’s vaccine uses synthetic Ribonucleic acid (RNA) messengers that use genetic code from the coronavirus to prompt human cells to generate a so-called ‘spike’ protein found on the outside of the virus. The process sets off an immune response from the body, which eventually blocks the actual coronavirus from latching onto cells.3
However, while requiring deep-frozen temperatures to retain efficacy, the Moderna vaccine only requires -20°C storage, as opposed to the -70°C required for the Pfizer-BioNTech vaccine.
Another benefit to the Moderna vaccine is that unlike the Pfizer-BioNTech vaccine that last for 5 days in refrigerated temperatures, the Moderna vaccine can retain its efficacy at 2°C – 8°C for 30 days, meaning a lot less pressure on the cold chain, and specifically end-user facilities worried about waste, or loss of efficacy before they have a chance to use it.
Like the Oxford-AstraZeneca vaccine, this feature could make the Moderna vaccine well suited to less well-connected parts of the global cold chain, including rural and less economically affluent areas.
Not just a box
One thing all three of the vaccines have in common is that they need to be transported in temperature-controlled packaging. Whether it’s -70°C, -20°C, or 2°C to 8°C the vaccine will need to move from manufacturer, to distribution hub, to regional end user facilities in packaging that can keep its contents within these temperature ranges for days at a time, and through extreme external temperature conditions.
The makeup of temperature-controlled packaging does pose some environmental challenges for a global vaccination program on this scale. In most cases, temperature-controlled packaging is comprised of these key material elements: cardboard or correx for the outer box, expanded polystyrene (EPS), or vacuum insulated panels (VIPs) as the insulator inside the box, and a phase change material (PCM) that will be dry ice for the Pfizer-BioNTech and Moderna vaccines, and water-based solution (potentially mixed with a chemical for improved performance) for the Oxford-AstraZeneca vaccine. There are additional materials used in specific cases, such as logging devices for tracking and tracing software that are often comprised of plastics and metals.
Getting effective COVID-19 vaccines to as many people as possible is undoubtedly, and rightly a global priority, however can we develop and implement solutions that achieve this goal while also minimising our global footprint?
Correx versus carboard
One way to reduce the impact of the vaccine cold chain on the environment is to look at cardboard alternatives. At Intelsius, we use correx in several products, including our ORCA range of temperature-controlled packaging. While correx isn’t widely recyclable in household recycling, it is a 100% recyclable material, and can be recycled at specialist recycling hubs subject to local guidelines.
But correx comes into its own in more ways than simply being 100% recyclable. One of the biggest advantages of correx is its durability, and therefore its longevity. Unlike cardboard, that becomes easily damaged after multiple use, and especially when it becomes wet, correx has a much longer shelf life. Its Polyethylene (PE) lining protects it from water and provides a much more robust outer container that’ll survive multiple journeys, meaning you’ll significantly reduce waste, and increase the reusability of your packaging.
VIPs versus EPS
Both vacuum insulated panels and expanded polystyrene are used to limit external temperature exposure, thus keeping your cargo at the desired temperature for longer.
Intelsius have ranges of products that use both VIPs and EPS as we look to balance value and performance for a range of customers. Both VIPs and EPS are recyclable (subject to local guidelines), however VIPs are the clear winner when it comes to environmental benefits.
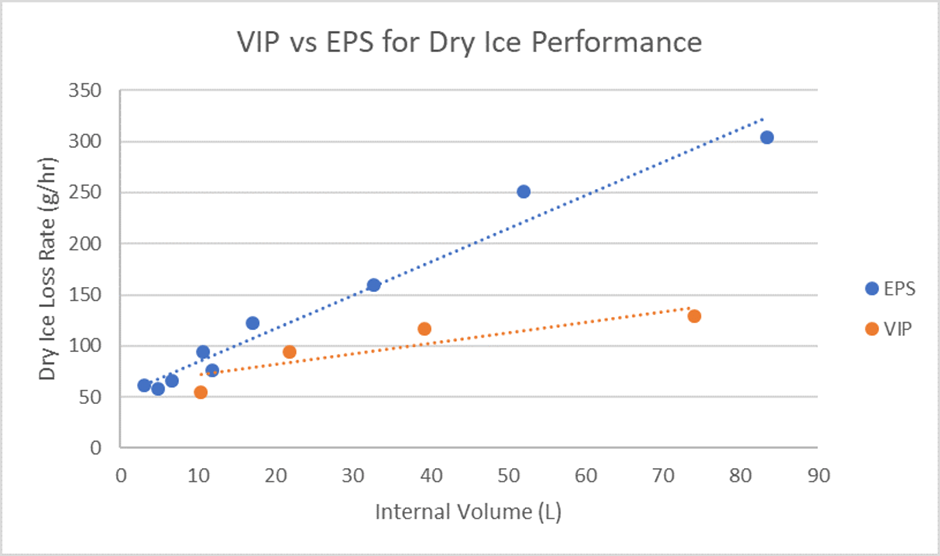
Simply put, vacuum insulated panels perform better than EPS when it comes to dry ice usage. A recent blog post exploring the role of dry ice in the vaccine cold chain explored the benefits of VIPs in the vaccine cold chain and is a handy resource for anybody currently assessing their packaging choices for vaccine shipments. The graph below outlines the key performance differences between EPS and VIPs when using dry ice as a PCM. As you can see, the loss rate of dry ice when using VIPs is significantly less than when using an EPS based product. This means better performance, and crucially in relation to the environment, less dry ice required.
Multiple-use and rental solutions
One way we can collectively reduce the impact of COVID-19 vaccine distribution on the environment is to look at reusable, or rental cold chain solutions. Reduction of waste is at the core of what we do at Intelsius, which is why our range of ORCA solutions are made available as single or multi-use. Made with a correx outer, and utilising vacuum insulated panels, the ORCA range is available with either dry ice for deep frozen shipments, or advanced phase change materials for refrigerated shipments.
Having a high-quality, multi-use system for vaccine distribution allows us to positively contribute to the global vaccination effort, while also ensuring our customers are reducing waste, without any drop-off in performance. To read more about, or purchase our range of ORCA multi-use systems, visit our website, or reach out to our customer services team.
Another solution to reduced wastage in the vaccine cold chain is rental services. Rather than having to purchase a box that you may only use once, or a handful of times, which may then ultimately end up taking space in your warehouse or office, or finding its way into landfill, our ORCA Fulfilment services allow you to access a high-quality temperature-controlled solution, without any lasting damage to the environment. To find out more about ORCA Fulfilment, visit our website, or reach out to our customer services team.
External References
1 – Science Focus (2020) – Everything you need to know about the Pfizer coronavirus vaccine
2 – BBC (2020) – Covid-19: Oxford University vaccine is highly effective
3 – Aljazeera (2020) – Moderna’s COVID-19 vaccine: What you need to know in 500 words