With the growing prospect of effective COVID-19 vaccines coming onto the market at any moment, cold chain solutions for their compliant shipping have become a priority for labs, drug suppliers, and health authorities everywhere.
According to the Financial Times1, clinical trials have sparked a flurry of multi-billion-dollar vaccine pre-orders from governments around the world, with biopharmaceutical company, Pfizer alone planning to deliver 1.2 billion doses of their RNA vaccine by the end of 2021. And, with the World Health Organisation reporting that there are 300 plus vaccines currently in clinical trials, we can expect global vaccine roll out for a variety of approved COVID-19 vaccines by the middle of next year.
The unprecedented scale of this undertaking has been further underlined by The International Air Transport Association (IATA) in a recent blog post2: ‘The potential size of the delivery is enormous. Providing a single dose to 7.8 billion people would fill 8,000 747 cargo aircraft. Even if we assume that half the needed vaccines can be transported by land, the air cargo industry will still face its largest single transport challenge ever.’
While the logistical planning and execution required for delivery on such a scale is complex enough, shipping COVID-19 vaccines comes with an additional challenge: the vaccines currently in development will need to be kept at specific temperatures, either between -20°C and -80°C, or between 2°C and 8°C, from filling , to the moment they arrive with an end user. As a result, keeping vaccines at the required temperature throughout the cold chain – thus ensuring efficacy – will not be possible without the use of temperature-controlled packaging, some of which will require dry ice as a coolant.
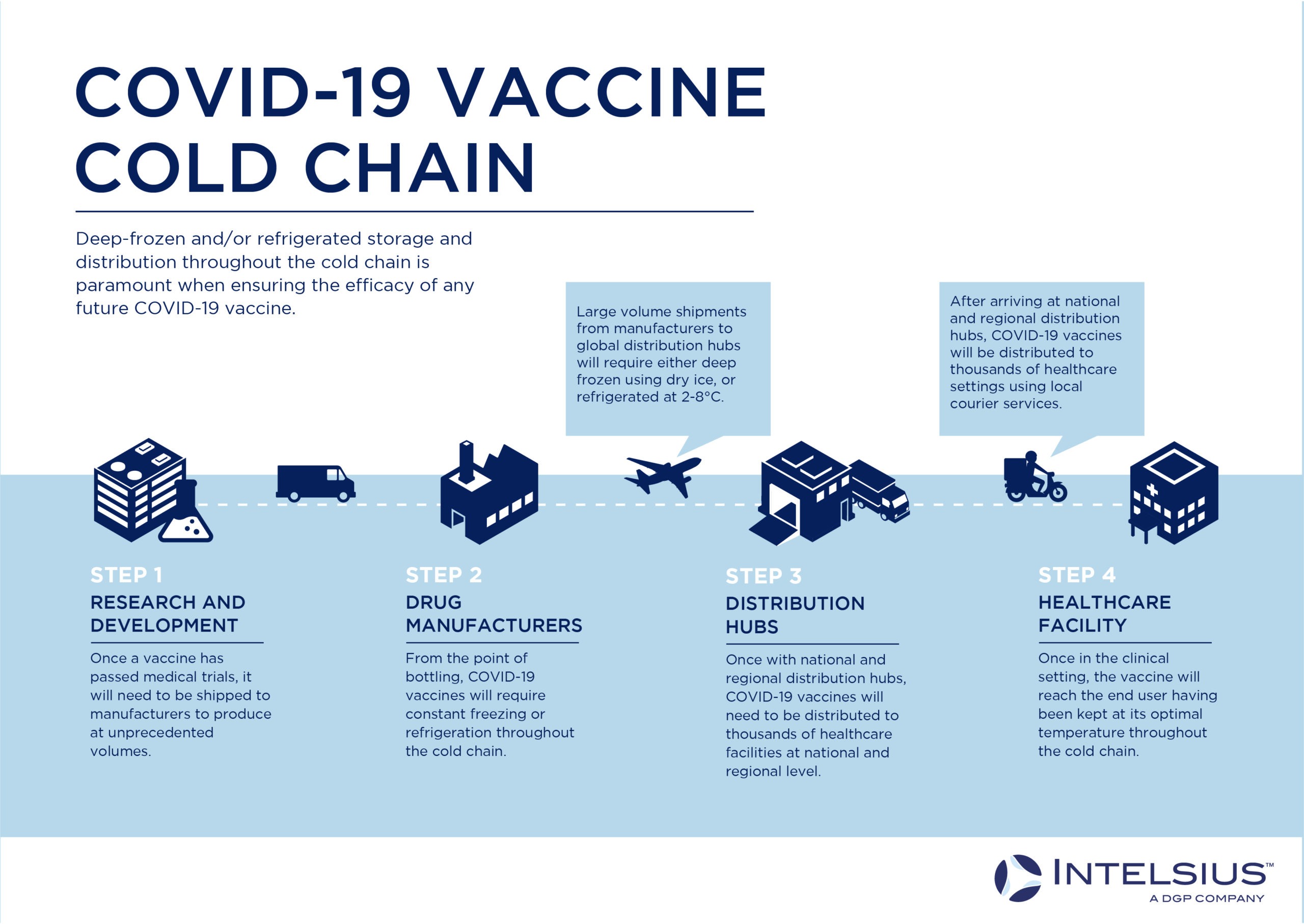
Infographic 1: Infographic showing COVID-19 Vaccine Cold Chain
The above infographic illustrates the various cold chain links required to get a COVID-19 vaccine from factory to end user. Though not all vaccines will need to be kept at sub-zero temperatures (some are likely to require 2-8°C refrigeration), for vaccines requiring between -20 and -80°C storage to retain efficacy (such as the recently phase 3 approved Pfizer RNA vaccine), the cold chain requires both deep freezer capabilities at storage and distribution centres, and specialist packaging to maintain these temperatures between storage centres. Ensuring the efficacy of your vaccines throughout the cold chain relies on effectively connecting those links in the cold chain with compliant temperature-controlled packaging solutions.
The Benefits of Dry Ice in the Cold Chain
Dry ice is the solid, frozen form of carbon dioxide and has two distinct advantages over other cooling agents in temperature-controlled packaging: it has a surface temperature of -109.3°F (-78.5°C), making it perfect for the shipment of biologics requiring sub-zero temperatures, and it sublimates (goes straight from solid to vapor), leaving behind no messy residue that could damage both the packaging and the cargo.
Combined with the right temperature-controlled packaging, it can provide the perfect cold chain shipping solution for COVID-19 vaccines and other vaccines and biologics requiring temperature stability at frozen temperatures.
However, sourcing dry ice has become more of a challenge for research and development companies currently developing a COVID-19 vaccine. With this important resource already in high-demand, ensuring we have solutions in place to meet the distribution demands of a worldwide roll out of one or more COVID-19 vaccines has become a top priority.
A Global Dry Ice Shortage
For several weeks it has been reported that the world is experiencing a dry ice shortage. One of the key drivers behind this shortage has been evident since the beginning of national lockdowns back in April 2020.
As mentioned above, dry ice is a solid form of carbon dioxide, a derivative of gases given off during the refinement of petroleum to gasoline and the burning of natural gas to produce ammonia for fertilisers, both of which have been in decline since the world’s manufacturing plants and roads went quiet last spring. While the reduced amount of CO2 being released into the atmosphere has environmental benefits, it also has a knock-on effect to numerous industries that rely on CO2, such as food and drink manufacturers, and crucially when it comes to vaccine development and distribution, dry ice producers.
With over 300 known COVID-19 vaccines either in pre-clinical, or phase 1-3 trials, the demand for dry ice has already increased markedly. Research and development companies around the world have an existing, urgent requirement for cold chain solutions that ensure their samples reach their destination with 100% efficacy.
Reduced availability of CO2, increased demand for dry ice from R&D companies around the world, and reduced capacity within logistics networks have all contributed to this shortage.
ORCA Solutions: Less Dry Ice, Superior Performance
ORCA solutions are a portfolio of high-performance, passive temperature-controlled packaging systems that use an innovative design, ensuring compliance and efficiency all whilst minimising wasted space and volumetric weight, leading to reduced shipment costs, and a reduced carbon footprint.
Available as either single-use, or multi-use systems, and available with real-time tracking capabilities, ORCA solutions combine dry ice and vacuum insulation panels, creating unparalleled performance when compared against other dry ice systems, ensuring costly temperature excursions are avoided.
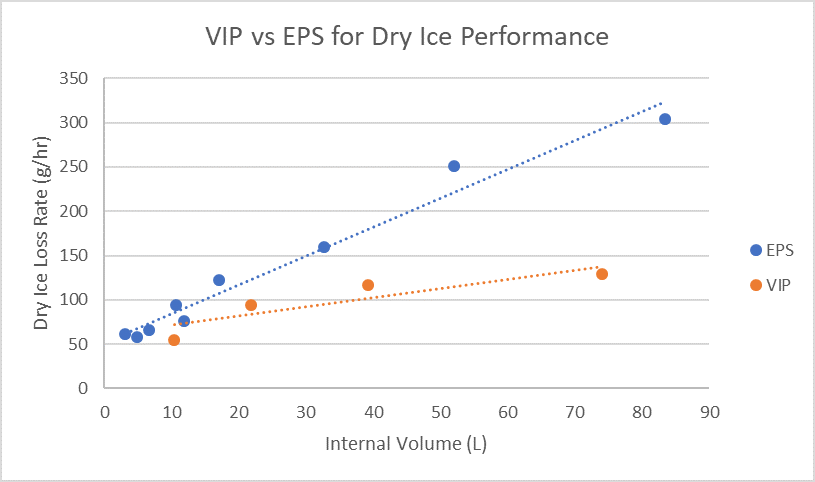
As illustrated in the above graph, this performance is achieved through interlocking VIPs that reduce the loss rate of dry ice, ensuring it can keep the payload at temperatures below -20°C for longer, giving your cargo 120+ hours of protection, versus the 96+ hours available with EPS based dry ice shippers. Finally, the ORCA dry ice range uses less dry ice, helping to support sustainable dry ice usage throughout this period of unprecedented demand.
Available in four different sizes, the Intelsius ORCA solutions provide payload volumes of between 1.5L and 50L, helping you ensure the efficacy and integrity of your cargo, anywhere in the vaccine cold chain.
Intelsius Founder & Chief Executive Officer, David P. Walsh outlines the potential role ORCA solutions could play in the delivery of COVID-19 vaccines: ‘We’re excited and proud that our ORCA systems can be a real solution to the impending global demand for compliant, deep frozen and refrigerated packaging systems throughout the vaccine cold chain. Delivering billions of COVID-19 vaccine doses around the world is one of the biggest and most important challenges of our lifetime, and our ORCA systems are here to help ensure that happens while ensuring the efficacy of the vaccines inside’.

Key features:
- Superior dry ice shipper solutions using VIP insulation
- Qualified against ISTA 7D profiles for a minimum performance of 120 hours
- Superior insulation leading to a reduction in dry ice requirements
- Multi-Use solutions available reducing packaging waste
- Connected solutions available providing peace of mind through payload visibility
Offering optimum protection for:
- Vaccines
- Active pharmaceutical ingredients
- Reagents
- Personalised medicines
- High-value temperature sensitive materials
For more information about our ORCA solutions visit: https://intelsius.com/products/temperature-controlled-packaging/
Or consult our ORCA Dry ice solutions product sheet
Alternatively, find your local branch and get in touch to discuss your temperature-controlled packaging requirements here: https://intelsius.com/contact-us/
External References
1 – Financial Times (2020) – How close is a coronavirus vaccine
2 – IATA (2020) – The Time to Prepare for COVID-19 Vaccine Transport is Now